Luo Lab research published in the EMBO Journal
01-07-2020
Luo Lab research published in the EMBO Journal
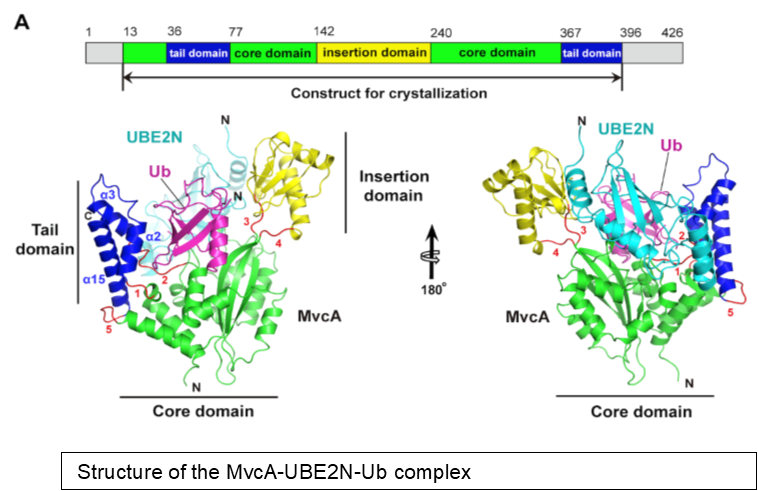
Ubiquitination involved in the attachment of the 76-residue ubiquitin protein to target proteins play essential roles in many cellular processes, particularly immunity. Pathogens have evolved various effective strategies co-opt the host ubiquitin network for their benefit. The intracellular bacterial pathogen Legionella pneumophila utilizes multiple virulence factors to hijack the host ubiquitin network. Earlier work from the Luo Lab shows that the virulence factor MavC induces monoubiquitination of UBE2N, an E2 conjugation enzymes that specifically directs the biosynthesis of K63-type polyUb chains, which are essential in activation of immunity. MavC accomplishes UBE2N ubiquitination by an unusual mechanism: It induces the formation of an isopentide bond between Q40 of ubiquitin and K92 of UBE2N by a transglutamination reaction that does not require exogenous energy input (Gan et al., Nature Microbiology, 2019). In the new study, Luo Lab found that MvcA, a close homolog of MavC (50% identity in primary sequences) function as a deubiquitinase that removes ubiquitn from UBE2N-Ub. Structural analysis of the MvcA-UBE2N-Ub complex reveals that the insertion domain of MvcA is responsible for substrate recognition.
The elucidation of the biochemical functions of MvcA has provides leads to the development of target-specific anti-infective agents. Importantly, because MvcA and MavC are highly similar at both sequence and structural levels, yet they catalyze completely two opposite biochemical reactions, which provides a new avenue to investigate protein evolution. Furthermore, the activity of these proteins suggests the existence of eukaryotic proteins capable of catalyzing ubiquitination by transglutamination.
Full citation: Gan N, Guan H, Huang Y, Yu T, Fu J, Nakayasu ES, Puvar K, Das C, Wang D, Ouyang S, Luo ZQ. Legionella pneumophila regulates the activity of UBE2N by deamidase-mediated deubiquitination. EMBO J. 2019 Dec 11:e102806. doi: 10.15252/embj.2019102806.
Contact: Zhao-Qing Luo: https://www.bio.purdue.edu/lab/luo/