A natural product chemical probe inhibits cardiac hypertrophy mediated by nuclear GPCR kinase 5
07-26-2019
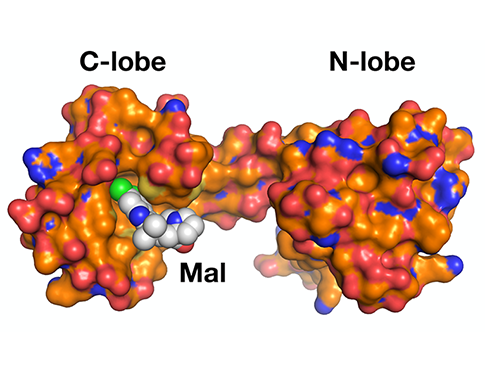
Tesmer lab research published in the Proceedings of the National Academy of Sciences
"G protein-coupled receptor kinase 5 is potently inhibited by the calcium-sensing protein calmodulin (CaM), which leads to nuclear translocation of their complex and activation of genes involved in cardiac hypertrophy. This study used small-angle X-ray scattering and negative stain electron microscopy to show that Ca2+·CaM binds primarily to the small lobe of the kinase domain of GRK5 near elements critical for receptor interaction and membrane association, thereby inhibiting receptor phosphorylation while activating the kinase for phosphorylation of soluble substrates. Use of a small molecule natural product known as malbrancheamide, it was shown that the C-terminal lobe of Ca2+·CaM regulates membrane binding while the N-terminal lobe regulates receptor phosphorylation and kinase domain activation. In cells, this chemical was able to halt GRK5 nuclear translocation and the resulting hypertrophic response."