Fat molecules influence form and function of key photosynthesis protein
09-30-2014
A mysterious space within a protein critical to photosynthesis is filled with fat molecules that influence both the protein's architecture and electrical properties, according to two recent studies.
Researchers studied the atomic structure of, and electrical interactions within, the cytochrome bf complex, a protein complex central to the transport of electrons within membranes of a plant cell, a critical step in photosynthesis.
Photosynthesis is the process by which plants, algae and bacteria convert sunlight, carbon dioxide and water into chemical energy.
William Cramer, the Purdue University professor who led the studies, said the step in which electrons are transported in the cytochrome complex is one of the slowest steps in photosynthesis and is of particular interest to those involved in the effort to speed up the process.
"The ability to manipulate photosynthesis - to make it faster or more efficient - could improve some of the global issues we face," said Cramer, the Henry Koffler Distinguished Professor of Biological Sciences. "It could lead to crops that grow faster to help feed an increasing population, and it could lead to a source of clean energy. However, before we can meaningfully manipulate the proteins involved in photosynthesis, we need to understand their structure in detail."
Cramer and S. Saif Hasan, a graduate student whose doctoral thesis work laid the foundation for this project, used high-resolution X-ray crystallography to reveal that a cavity within the protein was filled with lipids, greasy molecules commonly called fats. The team found a total of 46 lipid-binding sites within the protein, and the discovery could change the way the biophysics-biochemistry community thinks about membrane proteins, Cramer said.

"It had been known that lipids created a boundary around the outside of membrane proteins, but finding them inside the protein could shift our thoughts on how these complexes work," he said. "These lipids must be there for a reason, and we are trying to determine exactly what that is."
The researchers suggest the lipids help stabilize the structure of the protein, assist in the formation of super-complexes that combine multiple protein complexes, and influence the ability to move an electric charge between different portions of the protein. A manuscript detailing these findings was published in the journal Structure.
Cramer, Hasan and Stanislav D. Zakharov, a senior research associate in Cramer's laboratory, then collaborated with Sergei Savikhin, an associate professor of physics, and graduate students Adrien Chauvet and Valentyn Stadnystskyi to examine the electrical interactions of the protein complex.
Through a new spectrophotometric technique that simultaneously measures both electron transfer within the cytochrome complex and strength of interactions that result from the transfer, the team discovered that the electrical interaction varied between the different parts of the protein complex involved in transporting electrons, called hemes.
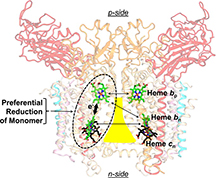
The lipid-filled cavity, roughly shaped like a triangle with a bottleneck, divides the two halves of the protein complex, and each half contains two hemes. Two hemes sit across from each other near the top of the cavity's narrow neck, and the other two sit across from each other near its wide bottom. The researchers found that although the two hemes at the top are closer together, the preferred electron transfer was to the heme on the same side of the protein complex.
It had been thought that the internal environment of membrane proteins was characterized by an equal and small dielectric constant, a measure of the ability to store electrical energy in an electric field. However, electron transport preferentially going to the heme on the same side of the cavity is only possible if there is a relatively large dielectric constant that directs the electron that way, Cramer said.
The expected value for the dielectric constant was around 2.5, but the researchers found that dielectric constants between the hemes ranged from 4 to 44 under different experimental conditions.
"This dielectric heterogeneity was a surprise," Cramer said. "If it varies among the different hemes, there are functional consequences, and we plan to explore this."
The lipid that separates the hemes and two halves of the protein complex likely deters electron transfer in that direction because of its small dielectric constant, but this doesn't fully explain the size of the differences, he said.
Zakharov refined the spectrophotometric technique, which uses changes in light absorption to determine the location of electrons. When different parts of the protein complex interact, unique signals are generated and the height of the signal is an indication of the strength of the interaction, Cramer said. This information, coupled with knowledge of the structure from Hasan's studies, allowed the team to determine the dielectric constants.
The collaboration between biophysicists and physicists was essential to this project, Cramer said. A paper detailing the technique and findings was published in the Journal of Physical Chemistry B.
The National Institutes of Health and Department of Energy funded this research.
Hasan and Chauvet earned their doctoral degrees after this research was completed. Hasan is currently a postdoctoral researcher studying virus structure in the laboratory of Michael Rossmann, Purdue's Hanley Distinguished Professor of Biological Sciences. Chauvet is now a postdoctoral researcher at the Laboratory of Ultrafast Spectroscopy at the Federal Polytechnical Institute in Lausanne, Switzerland.
Writer: Elizabeth K. Gardner, 765-494-2081, ekgardner@purdue.edu
Sources: William A. Cramer, 765-494-4956, waclab@purdue.edu
Sergei Savikhin, 765-494-3017, sergei@purdue.edu
Related news releases:
Purdue professor identifies proton pathway in photosynthesis
Purdue biologists crystallize technique to expand protein research
Purdue biologists' spotlight solves mysteries of photosynthesis, metabolism
ABSTRACT
A Map of Dielectric Heterogeneity in a Membrane Protein: the Hetero-Oligomeric Cytochrome b6f Complex
S. Saif Hasan, Stanislav D. Zakharov, Adrien Chauvet, Valentyn Stadnytski, Sergei Savikhin and William A. Cramer
The cytochrome b6f complex, a member of the cytochrome bc family that mediates energy transduction in photosynthetic and respiratory membranes, is a hetero-oligomeric complex that utilizes two pairs of b-hemes in a symmetric dimer to accomplish trans-membrane electron transfer, quinone oxidation-reduction, and generation of a proton electrochemical potential. Analysis of electron storage in this pathway, utilizing simultaneous measurement of heme reduction, and of circular dichroism (CD) spectra, to assay heme-heme interaction, implies a heterogeneous distribution of the dielectric constants that mediate electrostatic interactions between the four hemes in the complex. Crystallographic information was used to determine the identity of the interacting hemes. The Soret band CD signal is dominated by excitonic interaction between the intramonomer b-hemes, bn and bp, on the electrochemically negative and positive sides of the complex. Kinetic data imply that the most probably pathway for transfer of the two electrons needed for quinone oxidation-reduction utilizes this intramonomer heme pair, contradicting the expectation based on heme redox potentials and thermodynamics, that the two higher potential hemes bn on different monomers would be preferentially reduced. Energetically preferred intramonomer electron storage of electrons on the intramonomer b-hemes is found to require heterogeneity of interheme dielectric constants. Relative to the medium separating the two higher potential hemes bn, a relatively large dielectric constant must exist between the intramonomer b-hemes, allowing a smaller electrostatic repulsion between the reduced hemes. Heterogeneity of dielectric constants is an additional structure-function parameter of membrane protein complexes.
The Journal of Physical Chemistry
ABSTRACT
Internal Lipid Architecture of the Hetero-Oligomeric Cytochrome b6f Complex
S. Saif Hasan and William A. Cramer
The role of lipids in the assembly, structure, and function of hetero-oligomeric membrane protein complexes is poorly understood. The dimeric photosynthetic cytochrome b6f complex, a 16-mer of eight distinct subunits and 26 transmembrane helices, catalyzes transmembrane proton-coupled electron transfer for energy storage. Using a 2.5 Å crystal structure of the dimeric complex, we identified 23 distinct lipid-binding sites per monomer. Annular lipids are proposed to provide a connection for super-complex formation with the photosystem-I reaction center and the LHCII kinase enzyme for trans-membrane signaling. Internal lipids mediate crosslinking to stabilize the domain-swapped iron-sulfur protein subunit, dielectric heterogeneity within intermonomer and intramonomer electron transfer pathways, and dimer stabilization through lipid-mediated intermonomer interactions. This study provides a complete structure analysis of lipid-mediated functions in a multi-subunit membrane protein complex and reveals lipid sites at positions essential for assembly and function.
Structure